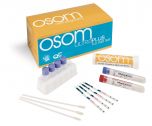
Quidel QuickVue Influenza A/B Tests
(25 Tests)
FREE Next Day Air Shipping on 4 or More Kits!
QuickVue Influenza (FLU) A+B Test Kits (dipstick format), allows for the rapid, qualitative detection of influenza type A and type B antigens directly from nasal swab, nasopharyngeal swab,nasal wash and or nasal aspirate specimens. FDA Cleared and CLIA Waived.
NOT APPROVED FOR HOME USE!
Now available for immediate sale.
QuickVue Influenza A+B Test:
The QuickVue Influenza A+B test allows for the rapid, qualitative detection of influenza type A and type B antigens directly from nasal swab, nasopharyngeal swab,nasal wash and/or nasal aspirate specimens. The test is intended as an aid in the rapid differential diagnosis of acute influenza type A and type B virus infection.
The QuickVue® Influenza test is a simple, 3-step, 1-reagent, 10-minute test that requires less than 90 seconds hands-on time. The test has a shelf life of 24 months from date of manufacture and can be stored at room temperature. No additional instrumentation is required to perform the test. The QuickVue® test is the first CLIA-waived rapid influenza test. The QuickVue® Influenza test combines speed, simplicity and accuracy all in one test.
Feature/Benefit:
- Differentiated results for type A and type B
- A positive diagnosis for influenza A can save $40 in prescription drug costs.**
** Cheung, M., M.D. and Lieberman, J.M., M.D., Influenza Update on Strategies for Management, Cont. Ped., Oct. 2002.
- A positive diagnosis for influenza A can save $40 in prescription drug costs.**
- Provides A or B results from one test procedure
- No redundant procedure required for differentiated results
- Two-color Result
- Easy to read test results. Decreased risk of misinterpretation.
- 10 Minutes to Result
- Allows prompt diagnosis and treatment while patient is still in office. Increases office efficiency and reduces patient follow up activities.
- 3 Step Procedure
- Fewer procedural steps. Decreased risk of operator error.
- Pictorial Procedure Guide
- Simplifies understanding of testing procedure. Increases reliability.
- Room Temperature Storage
- No reagent warm-up. Use as needed without waiting. Does not take up refrigerator space.
- 12-18 Month Dating*
- Long shelf life. Economical test for low & high volume labs.
- Built-in Internal Control
- Ensures system integrity.
- Kit Includes External Controls
- Facilitates internal laboratory quality control. No additional ordering needed.
CPT CODE*: 87804QW - Influenza A immunoassay, direct optical observation.
Reimbursement: $16.08
*All CPT codes are supplied for information purposes only and represent no statement; promise or guarantee by CLIAwaivedTM Inc. that these codes will be appropriate or that reimbursement will be made. It is the responsibility of the service provider to confirm the appropriate coding required by their local Medicare carriers, fiscal intermediaries and commercial payors.
NOTE: Not approved for home use.