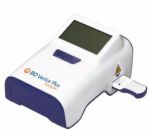
Point-of-Care Immunoassay Analyzer InfoScan Moduleritor, CLIA Waived
SKU
BD-256068
$499.50
(1 Module for BD Veritor™ System Test Devices)
Features
- CLIA-waived digital immunoassay for rapid detection of Flu A+B, RSV and Group A Strep
- Advanced particle technology enhances sensitivity by using a proprietary process to produce highly stable, modifi ed colloidal metal particles, helping improve test performance
- Adaptive read technology helps improve specificity to reduce false-positive results by compensating for background and non-specific binding
- Fast and accurate assay performance— after 10 minute incubation for Flu A+B and RSV; after 5 minute incubation for Group A Strep
- Prefilled color-coded unitized tubes by reagent facilitate workflow
- Swab or liquid sample is inserted into unitized tube, processed and removed
- Test device may be inserted immediately into Analyzer (Walk Away mode) or after incubation time is complete (Analyze Now mode)
- Print-enabled through USB
- BD Veritor™ InfoScan module facilitates traceability
- Reduces hands-on time through automated documentation
- Documentation functionality including operator ID, specimen ID, and test kit lot to enhance quality control
- Local data storage and download options for improved traceability
- BD Veritor is not included, this is a module
Product Specifications
| Manufacturer # | 256068 |
| Brand | InfoScan Moduleritor |
| Manufacturer | BD Primary Care |
| Country of Origin | China |
| Application | Point-of-Care Immunoassay Analyzer |
| CLIA Classification | CLIA Waived |
| Contents1 | Module Only |
| Dimensions | 7.6 X 9 X 14.3 cm |
| For Use With | For BD Veritor™ System Test Devices |
| Power Source | 100 to 240 VAC at 50 to 60 Hz, 0.6A |
| Readout Type | Digital Readout, Printout (Option) |
| Sample Type | Throat Swab / Nasal Swab / Nasopharyngeal Swab / Nasopharyngeal Aspirate or Wash Sample |
| Test Name | Influenza A + B, Respiratory Syncytial Virus (RSV), and Group A Strep |
| Time to Results | 5 to 10 Minute Results |
| UNSPSC Code | 41116144 |
| User Interface | LCD Display |
| Weight | 350 g |