DPP HIV 1/2 Assays
SKU
CHEMBIO-65-9500-0
$170.50
(20 Tests)
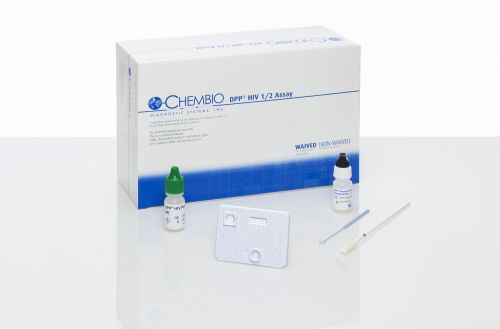
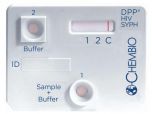
DPP HIV 1/2 Assay. Blood and Oral fluid test. CLIA waived, FDA approved.
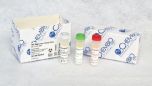
1 kit contains:
- 20 test devices
- 20 swabs
- 20 sample loops
- 20 specimen containers
- 1 running buffer
- 1 quick reference guide
- 20 subject information notices
- 1 product insert
NOT APPROVED FOR HOME USE - MEDICAL PROFESSIONALS ONLY.
CHEMBIO REIMBURSEMENT: CodeMap-Medicare Reimbursement Information
Chembio has developed a NEXT GENERATION DPP® assay using patented DPP®technology for the rapid detection of HIV 1 and HIV 2 antibodies in oral fluid and all blood matrices. The Chembio DPP® HIV 1/2 Assay is intended for use as a point-of-care test to aid in the diagnosis of infection with HIV 1 and HIV 2. This test is suitable for use in multi-test algorithms that have been established in many countries or as a standalone initial test.
For Use With:
- Oral fluid sample
- Venipuncture whole blood
- Serum
- Plasma
- Fingerstick whole blood
Assay Features:
- Sharp & distinctive visual results
- Excellent sensitivity and specificity
- Built-in procedural control to human IgG
- DPP®SampleTainer® bottle: a safe, closed system for handling potentially infectious blood and oral fluid samples
Convenient & Cost Effective
- Ideally suited for field and point-of-care testing
- Minimal sample size required, 10 µL
- Room temperature storage and procedure
- Ready-to-use reagents
- 24-month open vial stability for quality controls, 50 control runs per vial
- Long shelf life (23 months from date of manufacture)
- No special laboratory equipment required