Binx iO Molecular Point-of-Care Platform Instrument
SKU
BNX-3.001.001
Special Price $10,995.00 Regular Price $13,000.00
(1 Binx io Instrument with starter Kit)
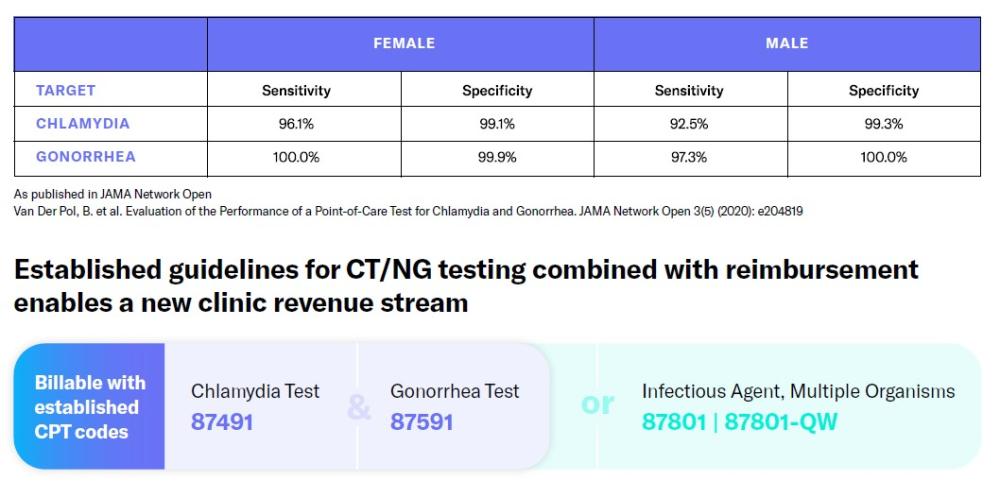
The binx io is a CLIA-waived, point-of-care PCR test for CT/NG (males & females) that delivers confirmed results in 30 minutes. This enables true test-and-treat in a single visit, improving antibiotic stewardship and quality scores.
The first CLIA-Waived molecular point-of-care solution for the rapid detection of chlamydia & gonorrhea!
With CLIA Waiver, the binx io expands access to laboratory-based quality testing in near-patient settings holding CLIA-certificates of waiver such as primary care offices, urgent cares, community clinics, emergency rooms, and retail pharmacies!
Ask about free analyzer with signed commitment contract of 25 tests per month!
- Contact [email protected] for details!
- First ever FDA 510(k), CLIA-Waived CT/NG molecular test for males and females, enabling same-visit diagnosis and treatment
- Gives results comparable to a laboratory-based test for chlamydia and gonorrhea in about 30 minutes rather than days or weeks
- Easy to use desktop-sized instrument that can be operated by non-laboratory trained personnel in CLIA-Waived settings
- Potential financial benefits for your healthcare practice