Accula‚™ SARS-CoV-2 Tests
SKU
MB-COV4100
(25 Tests per Kit)
Item Discontinued!
SUGGESTED REPLACEMENTS TO ACCULA PLATFORM:
Contents & storage
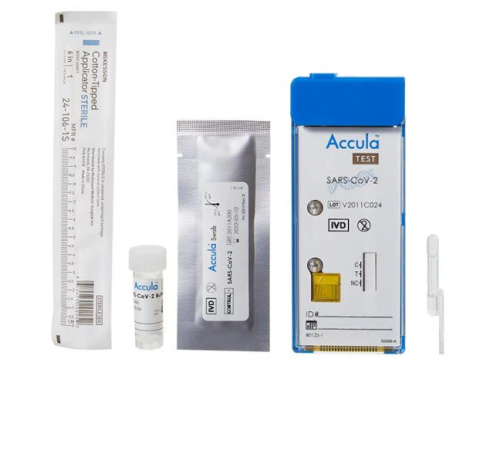
• Collection swabs (25): sterile swabs for nasal sample collection
• SARS-CoV-2 buffer (25): single-use vial of solution containing 5 mL of buffer with dimethyl sulfoxide and < 0.01% sodium azide
• Transfer pipette (25): single-use, fixed volume pipette used to transfer sample from the SARS-CoV-2 Buffer vial into the test cassette. Extra pipettes provided for your convenience.
• Accula SARS-CoV-2 test cassette (25): single-use, foil-pouched with desiccant and test cassette containing lyophilized reagents for the targeted amplification and detection of viral nucleic acid
• SARS-CoV-2 high positive, low positive, and negative control swab (1 each): DNA-based synthetic oligo dried onto a swab well above the limit of detection of the test
• SARS-CoV-2 buffer (25): single-use vial of solution containing 5 mL of buffer with dimethyl sulfoxide and < 0.01% sodium azide
• Transfer pipette (25): single-use, fixed volume pipette used to transfer sample from the SARS-CoV-2 Buffer vial into the test cassette. Extra pipettes provided for your convenience.
• Accula SARS-CoV-2 test cassette (25): single-use, foil-pouched with desiccant and test cassette containing lyophilized reagents for the targeted amplification and detection of viral nucleic acid
• SARS-CoV-2 high positive, low positive, and negative control swab (1 each): DNA-based synthetic oligo dried onto a swab well above the limit of detection of the test
Specifications
| Certifications/Compliance: | FDA Emergency Use Authorized (EUA) |
|---|---|
| Clia Complexity: | Waived |
| Control Sets: | High, Low, Negative |
| DoA Calibrators: | No |
| Final Product Type: | In vitro diagnostic |
| Format: | Test Cassette Kit |
| Kit Size: | 25 |
| Organism Group: | Virus |
| Sample Type: | Nasal swab |
| Sensitivity: | Product LoD is 4.75 x 102 NDU/mL |
| Shelf Life: | Minimum of 6 months from date of manufacture |
| Storage Requirements: | Room temperature |
| Technology: | RT-PCR, Rapid PCR |
| Test Time: | 30 minutes |
| Type: | SARS-CoV-2 Assay |
| CE Marker: | No |
| Detectable Analytes: | SARS-CoV-2 RNA |