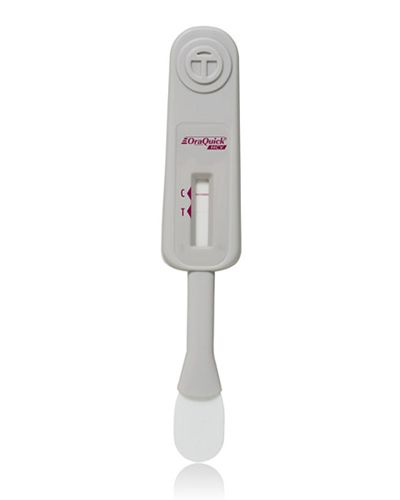
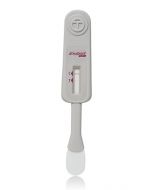
OraQuick HCV Rapid Antibody Test
(25 Tests)
Now CLIA-waived! The OraQuick® HCV test is the FIRST FDA approved test for detecting HCV antibodies in fingerstick and venipuncture whole blood. The simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes.
The OraQuick HCV Rapid Antibody Test is used to detect a patient’s exposure to the hepatitis C virus (HCV). Antibodies are proteins produced by the body to fight against foreign substances, such as viruses and bacteria. The presence of the antibodies associated with HCV (anti-HCV antibodies) can help determine if a person is currently infected or has previously been infected with HCV.
A person’s blood sample is added to a tube (vial) containing the test chemicals. The test strip is coated with HCV antigens (proteins from the HCV) and is placed into the vial. If there are antibodies to the HCV in the blood, they stick to the test strip and react with the chemicals to produce a colored line on the test strip. The appearance of a line indicates the presence of anti-HCV antibodies in the patient’s blood. A positive test result should be confirmed with a supplemental test as false positive results may occur.
This is the initial laboratory test used for people with signs or symptoms, or who are at risk of having HCV infection.
The test result is used in combination with other clinical information and blood tests to aid in the diagnosis of individuals with signs or symptoms of hepatitis, or in individuals at risk for hepatitis C infection.
- Accuracy: Clinically proven performance, the OraQuick® HCV is greater than 99% accurate.
- Appropriate For Testing In: OraQuick® is CLIA-waived and is appropriate for testing in locations such as Laboratories, Emergency Rooms, and Accidental Exposure Situations
- Clinically proven performance: The OraQuick® HCV is greater than 99% accurate.
FEATURES & BENEFITS:
- Easy-to-Use
- Greater Than 99% Accurate, comparable to laboratory-based tests
- Test Results in 20 Minutes
- Fingerstick and Venipuncture Whole Blood Sample Collection
- Tests for Multiple HCV Genotypes
- 9-12-Month Shelf-Life from Date of Manufacture
- Portable
- CLIAwaived
- CPT CODE 86803QW
Hepatitis C Virus Antibody
OraQuick HCV Rapid Antibody Test and Oraquick HCV Visual Reference Panel, Orasure Technologies, 86803QW
NOTE: This test should not be used to make a final diagnosis of HCV infection. If this test is positive, more tests will be required to determine if the person is infected. This test should not be used for screening patients without signs or symptoms of HCV or for blood donors, because it has not been proven effective for these purposes. The test should be used only when prescribed by a physician.
For training info, visit this link. For the implementation guide, click here.